O-PDX Pharmacology Study Predicts Drug Resistance, Leads to Compassionate Use of IGF-IR Inhibitor
Rachel, a previously healthy, 46-year-old woman, visited her family doctor in October of 2014, concerned about pain in her right chest wall. Her doctor ordered a CT scan, which revealed a 5.6 cm soft tissue mass with bony destruction in her eleventh rib; she was referred to an oncologist. A biopsy found that Rachel had a rare tumor in the Ewing Sarcoma family.
Genetic Profiling Yields No Leads
Genetic sequencing performed by a third party identified a FUS-ERG gene translocation and CDKN2A mutation in Rachel’s tumor—a condition so rare that bioinformatics algorithms often used to suggest potentially efficacious drugs returned no results.
Rachel’s oncologist, a prominent Sarcoma specialist, treated her with two rounds of vincristine, doxorubicin (Adriamycin), and cyclophosphamide (VDC)—the first-line standard of care for Ewings tumors. Several weeks later, she underwent surgery, removing pieces of rib bone, abdominal wall muscle and diaphragm. Certis orthotopically engrafted this tissue in immunocompromised mice, creating O-PDX replicates of Rachel’s cancer in preparation for a pharmacology study.
The post-surgical pathology report indicated treatment with VDC had regressed Rachel’s tumors by 10% and that no cancer cells were found at the edge of the tissue (typically reported in medical terms as “negative margins”). Encouraged by this news, her oncologist recommended 12 cycles of VDC in combination with iFOSFamide, Etoposide, (VDC/IE). Seven months passed. A follow-up PET scan showed no evidence of disease. Rachel and her family were elated!
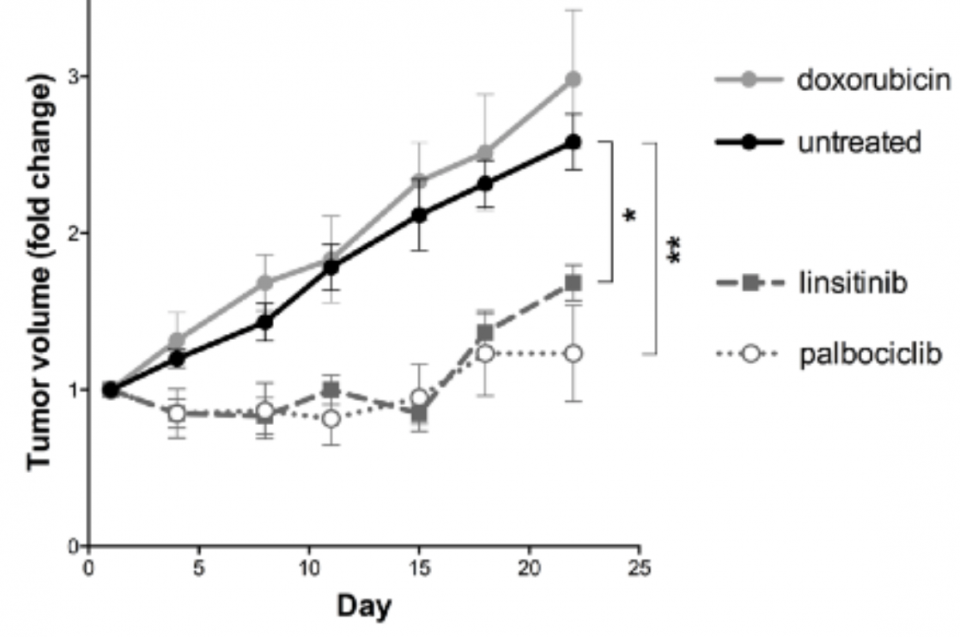
Simultaneous to Rachel’s treatment, Certis scientists had completed Rachel’s pharmacology study. The results raised concerns about drug resistance, as her O-PDX models had stopped responding to doxorubicin. But the study also gave insight into Rachel’s possible next line of treatment, as her mice also showed sensitivity to CDK4/6 and IGF-IR inhibitors.
In December 2016, Rachel again became ill, and a follow-up MRI confirmed that her aggressive cancer had returned and metastasized throughout her skeletal system, leading to severe cytopenia, a condition marked by deficiency of mature blood cells. Rachel’s oncologist drew on data from the O-PDX pharmacology study completed several months before, which showed sensitivity to other types of drugs. He used this data to request compassionate use of an IGF-IR inhibitor, ganiturnab.
As Rachel underwent treatment, Certis initiated a second O-PDX study, orthotopically engrafting fresh tumor tissue into a new group of mice. Her oncologist introduced four new test therapies in this second study. In four of the five arms, Rachel’s tumors grew rapidly. But one therapy–a combination of irinotecan and temozolomide—showed a significant reduction in tumor volume. This was all Rachel and her oncologist needed to move forward with certainty. Rachel began treatment in May 2017, and just as her mouse avatars had done, she responded very positively to irinotecan/temozolomide in the months ahead.
Elliott IA, Russell TA, Graham D, K Miyake, JG Crompton, Eckardt MA, Brackert S, Federman NC, Yanagawa J, Chmielowski BC, Hoffman RM, Eilber FC, Singh AS